There are more than 10 million smokers in Italy, representing about 20% of the population over 15 years of age. Many of them would like to stop smoking but most of the products to do so, present on the market, seem not to work properly.
Chrono Therapeutics has developed a wearable device that can analyze smoker behaviors, stress levels and routines.
With a basic schedule of just 10 weeks, just over 2 months, the smart device of the IoT world of health aims to make anyone stop smoking. To succeed, the device, connected to the appropriate app available for Android smartphones and iPhones, offers a range of tools that help smokers take away the vice to understand, manage and overcome one’s physiological desires caused by nicotine addiction. The latter is in fact the main active component at the neurological level of the cigarette, which causes a strong dependence in the subject that consumes it, stimulating the brain system.
In addition to physiological desires, psychological desireshave an important role in the vice of smoking, triggered by everyday events such as stress that increase when you are in the presence of other smokers or a simple cup of coffee, as you often accompany it with a cigarette.
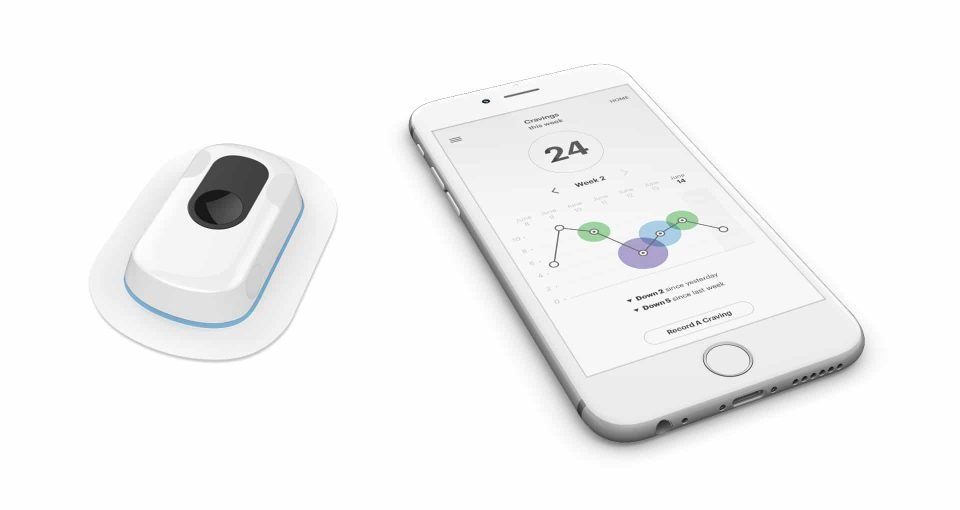
The dedicated application has a simple and intuitive interface, where it shows for example the level of smoking desire and graphs related to the vice of smoking, on your smartphone.
Usually the urge to smoke axle in the morning (75% of smokers smoke within 30 minutes of waking), in the evening before going to bed and immediately after meals, then it follows a more or less regular pattern, that you can notice thanks to the app.
Chrono then acts by regulating the supply of nicotine, through the dispenser, which is comfortable and light and can be applied to the skin discreetly, under the clothing. Thus the device collects all the necessary data, since every time the person has the desire to smoke, he must press the pulse of the dispenser, so that he stops smoking gradually, reducing More and more the nicotine level, for example from 21 mg to 7 mg, until it is completely eliminated.
The device is currently being approved by the Food and Drug Administration (FDA), the US government body dealing with food and pharmaceutical regulation.For more details Contact Us or Click Here !